Microfluidics is a new technology platform used to manipulate extremely small amounts of liquid (10-9~10-18L).
Microfluidic analysis is a micro-total analysis system that integrates sampling, dilution, reagent addition, reaction, separation, and detection functions onto a chip by connecting micro-pumps, micro-valves, micro-reservoirs, micro-electrodes, micro-detection elements, and other components with optical and charge fluid transport capabilities via a network of microchannels.
As a typical representative technology of lab-on-chip, microfluidic technology has developed rapidly and has now become an interdisciplinary field covering separation analysis, molecular biology research, and biomedical diagnosis.
Due to their micrometer-scale structures, fluids exhibit and generate special properties in microfluidic chips that differ from those at the macroscopic scale, thereby enabling the development of unique analytical capabilities.
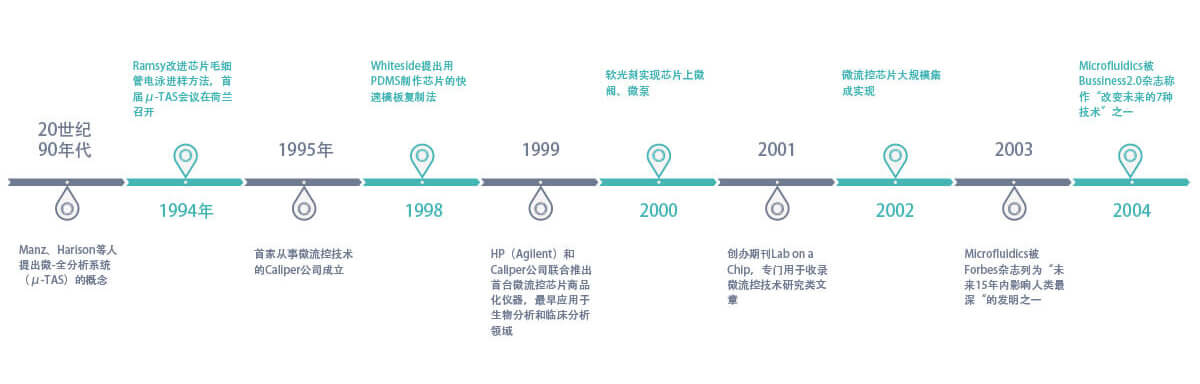
Development History
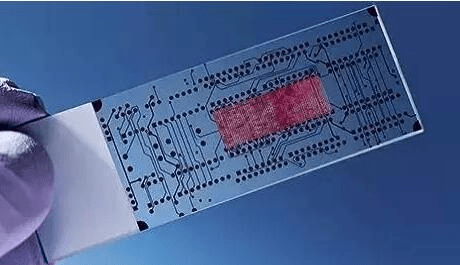
Manz and Widmer et al. first proposed the concept of a Miniaturized Total Analysis System (MTAS) in 1990. By 1995, Caliper Life Sciences, the first company specializing in microfluidic chip technology, was established. In the mid-1990s, the U.S. military's demand for portable biochemical self-testing equipment for individual soldiers spurred global research into microfluidic chip technology.
Throughout the 1990s, microfluidic chips were primarily regarded as an analytical chemistry platform. As such, microfluidic chips could theoretically serve as a “micro-total analysis” technology platform applicable to various analytical fields, including biochemical medical diagnostics, food and commodity inspection, military science, and aerospace science, among other critical application areas. Among these, biomedical analysis emerged as a key focus area.
Principle of microfluidic technology
Microfluidic chips utilize micro-electro-mechanical processing technology similar to that used in semiconductors to construct microfluidic systems on chips. This allows experimental and analytical processes to be transferred to a chip structure composed of interconnected pathways and liquid-phase chambers. After loading biological samples and reaction solutions, micro-mechanical pumps are employed.
Electrohydraulic pumps and electroosmosis are used to drive the flow of buffer solution in the chip, forming microfluidic channels, where one or multiple reactions can be performed on the chip.
Various detection systems, including laser-induced fluorescence, electrochemistry, and chemistry, as well as many detection methods combined with analytical techniques such as mass spectrometry, have been used in microfluidic chips for fast, accurate, and high-throughput analysis of samples.
Features of microfluidic technology
The most distinctive feature of microfluidic chips is their ability to form multifunctional integrated systems and numerous composite systems on a single chip, thereby enabling micro-total analysis systems.
Advantages of microfluidic technology
Microfluidic chips integrate a series of basic operational units—including sample preparation, reaction, separation, and detection—from fields such as chemistry and biology onto a single chip measuring just a few micrometers in size. The network of microchannels formed within the chip spans the entire system, offering advantages such as portability, low energy consumption, ease of fabrication, and ease of use. These features make microfluidic chips well-suited to meet the demands of life sciences for low-dose, more efficient, highly sensitive, and rapid separation and analysis of biological samples.
1. Integration of miniaturization and automation
Microfluidic technology integrates multiple steps of sample detection onto a tiny chip by combining the dimensions and curvature of channels, microvalves, and cavity designs to integrate these operational steps, ultimately enabling the entire detection process to be miniaturized and automated.
2. High throughput
Since microfluidics can be designed with multiple channels, a microchannel network can simultaneously divert samples to be tested into multiple reaction units, which are isolated from each other to prevent interference between reactions. This allows multiple tests to be performed in parallel on the same sample as needed.
Compared with conventional individual item testing, this method significantly reduces testing time, improves testing efficiency, and features high throughput.
3. Low consumption of testing reagents
Due to the miniaturization of integrated detection, the reaction chamber on microfluidic chips is very small. Although the concentration of reagent formulations may be increased by a certain percentage, the amount of reagents used is far lower than that of conventional reagents, greatly reducing reagent consumption.
4. Small sample size required
Since the detection is performed on a tiny chip, the sample volume required for detection is very small, often only microliters (μL) or even nanoliters (nL).
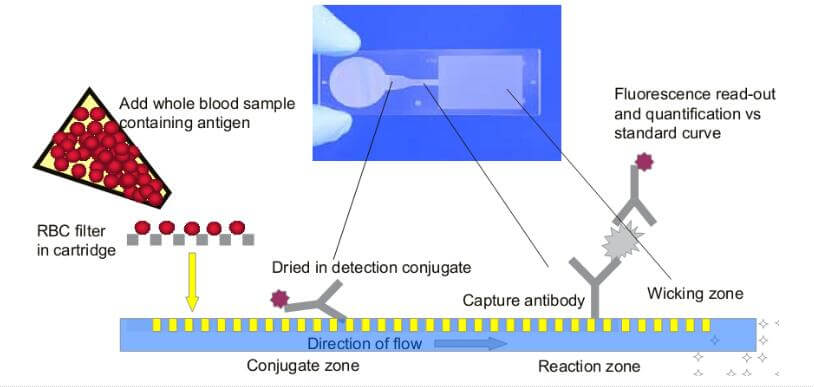
In addition, whole blood can be used directly for testing, making it more convenient for infants, the elderly, and people with disabilities who have low blood volume or difficulty with venous blood collection. Alternatively, it can be used for very precious or rare samples, enabling multiple indicator testing.
5. Low pollution
Due to the integrated functionality of microfluidic chips, all operations that previously required manual completion in the laboratory are now integrated onto the chip and performed automatically, minimizing sample contamination from the environment during manual operations.
Disadvantages and limitations of microfluidics
Lack of norms and standards for core technologies
Serious shortage of relevant talent
Currently, production costs are high.
Chip
Microfluidic chips were initially referred to as “lab-on-a-chip” in the United States and “micrototal analytical systems” in Europe. They serve as the primary platform for microfluidic technology, enabling the integration of fundamental operational units such as sample preparation, reaction, separation, and detection in biological, chemical, and medical analysis processes onto a single chip at the micrometer scale, thereby automating the entire analytical process.
The primary materials used in the fabrication of microfluidic chips include silicon wafers, glass, polydimethylsiloxane (PDMS), polymethyl methacrylate, polytetrafluoroethylene, and paper-based substrates.
Among these, PDMS has the widest range of applications. This material is not only easy to process and optically transparent, but also has a certain degree of elasticity, making it suitable for the production of functional components such as microvalves and microperistaltic pumps.
| Material types | vantage | drawbacks |
| monocrystalline silicon | Chemically inert and thermally stable; Mature processing technology, compatible with established processes such as photolithography and etching for fabricating integrated circuits, enabling processing and mass production; | Fragile and expensive; Not UV-transparent; Poor electrical insulation properties and complex surface chemistry; |
| Glass and quartz | Excellent electrolytic properties; Excellent optical properties; Surface modification can be performed using chemical methods; Processing can be carried out using lithography and etching techniques; | It is difficult to obtain channels with a large aspect ratio, resulting in high processing costs; Bonding is difficult; |
| organic polymers | Low cost, wide variety; Transparent to visible and ultraviolet light; Can be surface-modified using chemical methods, easy to process, and can be formed into deep channels with high aspect ratios via casting, laser sputtering, and other methods; Can be produced in large quantities at low cost; | Not resistant to high temperatures; Low thermal conductivity; Methods for surface modification require further research; |
| Polydimethylsiloxane (PDMS) | Capable of repeated reversible deformation without permanent damage, microfluidic chips can be prepared with high fidelity using molding methods. They are transparent to ultraviolet and visible light with wavelengths exceeding 300 nm, durable, chemically inert, non-toxic, and inexpensive. | Not heat resistant; Low thermal conductivity; Methods for surface modification require further research; |
Microfluidic chip fabrication
Different material properties determine different microfabrication methods. However, the primary fabrication methods for microfluidic chips are derived from photolithography technology in the microelectronics industry and soft photolithography technology from surface patterning.
1. Microfluidic chip processing
This step requires consideration of structural design, cost, pipe dimensions, and scalability for mass production. Current technologies include: lithography and etching techniques, hot pressing, molding, injection molding, LIGA (combining lithography, electroforming, and plastic casting), laser ablation, and soft lithography.
2. Microfluidic chip sealing
The issues to consider in this step include: high-temperature performance degradation, room-temperature aging, selection of point sealing or surface sealing, whether the pipe will become blocked, and whether mass production is feasible. The current technologies available include: plasma/ionization bonding, film lamination, ultrasonic welding, laser welding, and hot-press bonding.
3. Microfluidic fluid drive
The main considerations for this step include pumps and valves, including whether to choose active or passive types, and whether they are stable and reliable.
On the other hand, it is necessary to consider the width and depth of the fluid, the size of the chamber, and whether to use quantitative or qualitative analysis.
The current driving methods mainly include: light control, electric drive, magnetic field, vesicle squeezing, membrane vibration, pump pushing, centrifugal force, and shear force.
4. Aerosol pollution design
This step requires consideration of what materials or methods to use to minimize aerosol contamination.
The following methods can currently be used: sealing the reaction system before amplification, fully sealed system, silicone oil sealing, sealing the sample addition port after adding the sample, snap-on structure, and manual sealing.
5. Instrument signal detection
Microfluidic droplet signals are collected, involving the following main technologies: visualization readout, electrical signal readout, and amplification curves.
Applications of microfluidics in the field of in vitro diagnostics
Microfluidics is now a mature technology, with over a thousand companies worldwide developing microfluidics-based solutions.
In 2018, the global microfluidics product market reached US$8.7 billion, with a compound annual growth rate (CAGR) of 11.7% from 2019 to 2024, and is expected to reach US$17.4 billion by 2024. Microfluidic chip IVD products have disruptive advantages in certain aspects and are bound to develop into the mainstream in vitro diagnostic technology.
organ chip
An organ-on-a-chip is a scientific technology that simulates organ functions on a microfluidic chip platform. It was selected as one of the “Ten Emerging Technologies” at the 2016 World Economic Forum in Davos.
Its primary objective is to simulate the environment of biological organisms on a chip to conduct cell, tissue, and organ culture, study and control the biological behavior of cells during in vitro culture, thereby achieving organ transplantation and drug evaluation that mimic the environment of biological organisms.
Organ-on-a-chip technology enables the creation of complex systems, with clinical applications already available for kidney chips, liver chips, pancreatic chips, intestinal chips, vascular glucose chips, and tumor chips, among others.
liquid biopsy
Taking circulating tumor cell (CTC) detection as an example, it plays a significant role in tumor staging, dynamic monitoring, efficacy assessment, drug development, and prognostic evaluation. It represents a promising new liquid biopsy technology that could potentially replace tumor tissue biopsy.
However, current CTC immune enrichment and technical methods that rely on a single epithelial-derived antibody cannot comprehensively capture different subtypes of CTCs, are difficult to release CTCs without damage, and cannot provide in-depth molecular pathological information.
Using microfluidic technology, multiple nucleic acid sequences with high affinity and specificity for different CTCs can be obtained. By constructing microfluidic microcolumn array chips, CTCs can be efficiently captured and released without damage. This method has important application prospects in the precise diagnosis of cancer, drug guidance, and efficacy evaluation.
drug screening
Drug screening is a step in the modern drug development process for testing and obtaining specific physiologically active compounds. Microfluidic chip technology, with its characteristics of low sample consumption, high speed, high column efficiency, and solution systems that closely resemble biological fluids, has become a highly promising tool for the efficient screening of drugs and lead compounds.
© 2025. All Rights Reserved. 苏ICP备2022036544号-1